HumanKine® Cytokines & Growth Factors를 선택해야 하는 이유 6가지
20세기에는 DNA를 쓰고, 편집하고, 지울 수 있는 분자생물학의 획기적인 발전으로 인해 의학의 새로운 가능성을 만들었습니다. 주목할 만한 초기의 예로는 재조합 인슐린을 만들기 위해 Genentech 에서 사용한 E. coli 입니다.
생물학과 의학이 변화함에 따라 재조합 단백질의 요구 사항도 변화해왔습니다. 많은 단백질들은 activity를 위해 eukaryotic system에서만 가능한 glycosylation 및 processing이 필요했고, 이러한 이유로 곤충과 Chinese hamster ovary (CHO) 세포를 재조합 단백질 생산자로 개발하게 되었습니다.
과학과 의학이 진보함에 따라, 기존의 시스템들은 늘어나는 요구사항을 충족시키는데 한계를 겪고 있습니다. 예를 들어, human stem cell technology와 CAR-T cell therapy는 animal component 및 xenobiotic free 이면서도 높은 활성과 확장성을 가지는 culture media를 필요로 합니다.
Human applications 및 research에는 human cell expression system이 이상적입니다. Proteintech의 HumanKine® 재조합 단백질은 모두 HEK293 세포를 사용하여 만들어졌으며, 임상 연구의 새로운 요구에 완벽하게 부합합니다.
HumanKine® Advantages
Products derived from or by an animal are not used at any point during production.
Benefits of animal component free and xeno free proteins:
-
The final product does not contain any constituent that is either an animal tissue, body fluid or components derived from it.
-
All materials from procurement to final products are stored and handled in dedicated animal free facility.
-
Final product and the process does not involve the use of materials from non- human animal sources or recombinant materials made from non-human animal sources.
No affinity tags such as HIS tag or GST tag are used for purification as these tags may interfere with cytokine active site and bioactivity.
Benefits of tag free proteins:
-
No tags are used for expression and purification of proteins.
-
Inclusion of a tag can often result in changes to the structure of the protein of interest.
-
Sometimes a tag interferes with the active site of the protein resulting in altered biological activity.
-
Presence of a tag can increase immunogenicity of some protein, which makes a tag-free recombinant protein more desirable for in vivo applications.
Endotoxin: LAL tested, < 0.1 EU/µg
The purity of a recombinant protein has an influence on the overall quality of the final product. It is important to use proteins with the highest purity possible (ideally >95%) to ensure the product you use is as free from other extraneous proteins, endotoxins, or anything else that could interfere with the physiological mechanism of action of your recombinant protein. Endotoxins in commercially available recombinant proteins have been known to interfere with the biological activity of the protein, or the cultured cells. Endotoxins such as LPS can activate immune cells in culture; even at ‘commercially acceptable levels’. It is important that the recombinant protein you buy is endotoxin-free or has extremely low levels of endotoxins.
Native post translational modifications improve bioavailability in the cell culture media.
Though CHO and insect cells are eukaryotic, their ability to process human proteins does not match human cells in many cases. For example, Figure 1 shows that human cells generate more mature Activin A dimers than CHO cells.
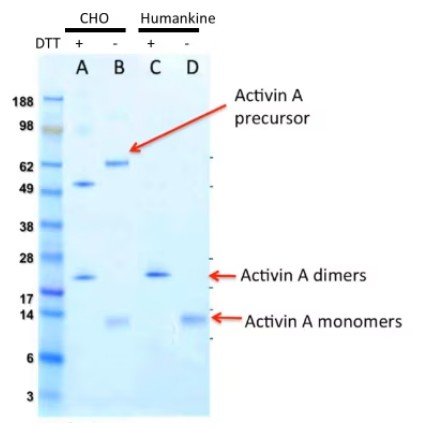
Fig 1. SDS-FAGE gel with Coomassie blue staining of purified Activin A from CHO cell and HumanKine® systems, demonstrating the formation of mature Activin A dimers.
Human-cell expression gives authentic human post-translational modifications and highest bioactivity.
Glycosylation is crucial to stability and activity. CHO and insect cells have vastly different machinery for this process, producing glycosylated species that can be very different from humans. Figure 2 contains an example where glycosylation differs between expression systems.
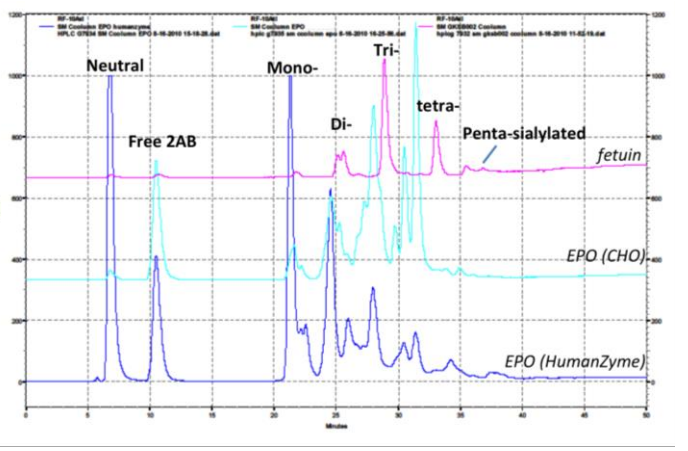
Fig 2. HPLC comparison of glycosylation of human cell expressed (Humanzyme) and CHO EPO, demonstrating significant differences in glycan composition. Fetuin was used as a positive control.
Due to native glycosylation and maturation, human cell expressed proteins can outperform other systems in terms of stability. Figure 3 shows how HumanKine FGF has greater stability in culture than an E. coli-derived FGF.
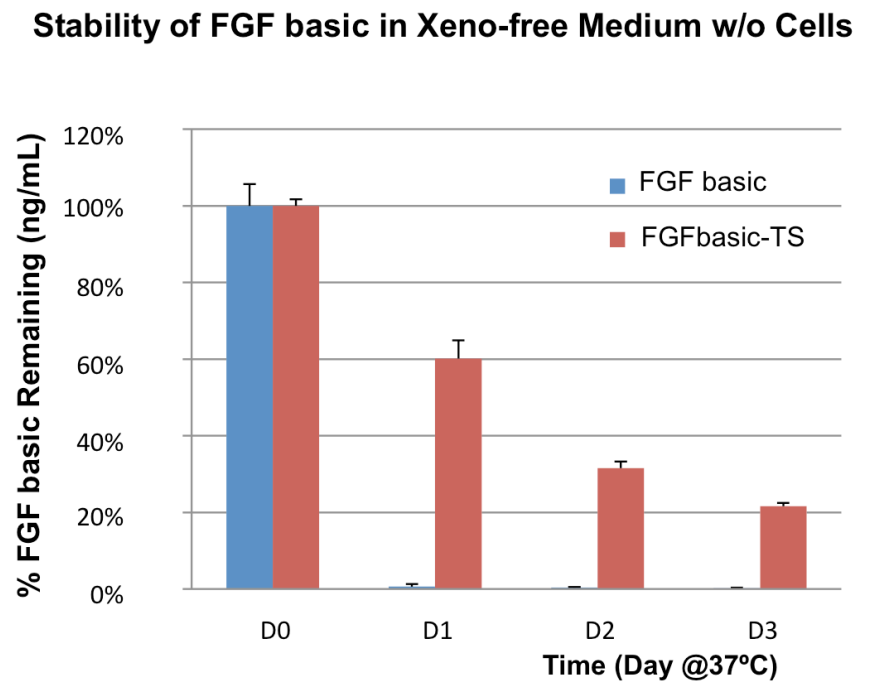
Fig 3. Comparision of stability of HumanKine® (FGF basic-TS, red) and E.coli-derived (FGF basic, blue) FGF incubated in cell culture at 37C without cells.
The above features synergize to create proteins that tend to have higher activity than those produced in other expression systems. Figure 4 compares activity for multiple cytokines between eukaryotic systems.
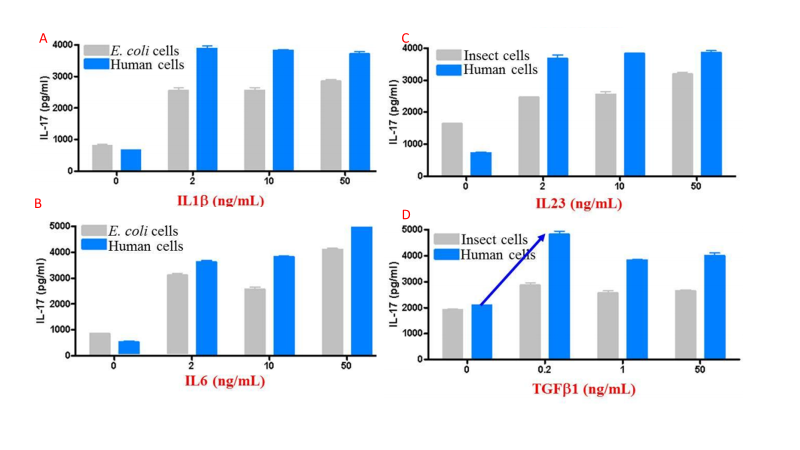
Fig 4. Comparison of activity of insect, human, and E. coli cell expressed cytokines (A, IL1B. B, IL6. C, IL23. D, TGFB1), determined by Th17 differentiation of human CDE4+ cells.
There is a common thread that unites many biotechnology stories: Discover a general principle in bacteria, then work up model organisms until ready to apply to human goals. Protein production follows this pattern and with HumanKine® products, we are finally at the stage of using human cells to make human proteins for human use.

With this certification, Proteintech’s HumanKine® Human cell-expressed cytokines and growth factors are now available in GMP-compliant versions for use in clinical trials and commercial manufacturing.
Research-grade Product list
요청 시, 모든 HumanKine Research-grade 제품들은 cGMP-grade로 제공 가능합니다. 이미 제품화된 cGMP-grade protein list는 상기 6번 항목의 링크를 클릭하시면 확인하실 수 있습니다. (예시; Activin A cGMP-grade / Cat. No. HZ-1138-GMP)
| Product Name | Catalog number | Activity spec. | Purity |
| Activin A | HZ-1138 | ≤5 ng/mL EC50 | >95% |
| beta NGF | HZ-1222 | ≤3 ng/mL EC50 | >95% |
| BMP-2 | HZ-1128 | ≤60 ng/mL EC50 | >95% |
| BMP-4 | HZ-1045 | ≤10 ng/mL EC50 | >95% |
| BMP-7 | HZ-1229 | ≤100 ng/mL EC50 | >95% |
| Cystatin C | HZ-1211 | ≤5 µM IC50 | >95% |
| EPO | HZ-1168 | ≤2.5 ng/mL EC50 | >95% |
| FGF Basic TS | HZ-1285 | ≤0.5 ng.mL EC50 | >95% |
| FGF-4 | HZ-1218 | ≤1.25 ng/mL EC50 | >95% |
| FGF-7 (KGF) | HZ-1100 | ≤7.5 ng/mL EC50 | >95% |
| FGF-8b | HZ-1103 | ≤10 ng/mL EC50 | >95% |
| FLT3 Ligand | HZ-1151 | ≤0.8 ng/mL EC50 | >95% |
| G-CSF | HZ-1207 | ≤0.1 ng/mL EC50 | >95% |
| GDNF | HZ-1311 | ≤ 10 ng/mL EC50 | >95% |
| GM-CSF | HZ-1002 | ≤0.5 ng/mL EC50 | >95% |
| HGF | HZ-1084 | ≤20 ng/mL EC50 | >95% |
| HGH | HZ-1007 | ≤0.5 ng/mL EC50 | >95% |
| HSA | HZ-3001 | N/A | >95% |
| IFN alpha 2A | HZ-1066 | ≤0.4 ng/mL EC50 | >95% |
| IFN alpha 2B | HZ-1072 | ≤0.12 ng/mL EC50 | >95% |
| IFN beta | HZ-1298 | ≤0.1 ng/mL EC50 | >95% |
| IFN gamma | HZ-1301 | ≤0.05 ng/mL EC50 | >95% |
| IL-1 beta | HZ-1164 | ≤0.05 ng/mL EC50 | >95% |
| IL-2 | HZ-1015 | ≤5 ng/mL EC50 | >95% |
| IL-3 | HZ-1074 | ≤2 ng/mL EC50 | >95% |
| IL-4 | HZ-1004 | ≤0.6 ng/mL EC50 | >95% |
| IL-6 | HZ-1019 | ≤0.5 ng/mL EC50 | >95% |
| IL-7 | HZ-1281 | ≤1 ng/mL EC50 | >95% |
| IL-9 | HZ-1240 | ≤1 ng/mL EC50 | >95% |
| IL-10 | HZ-1145 | ≤1.5 ng/mL EC50 | >95% |
| IL-12 | HZ-1256 | ≤2 ng/mL EC50 | >95% |
| IL-17 (IL-17A) | HZ-1113 | ≤2 ng/mL EC50 | >95% |
| IL-17F | HZ-1116 | ≤10 ng/mL EC50 | >95% |
| IL-23 | HZ-1254 | ≤4 ng/mL EC50 | >95% |
| IL-27 | HZ-1275 | ≤12 ng/mL EC50 | >95% |
| IL-28A | HZ-1235 | ≤5 ng/mL EC50 | >95% |
| IL-28B | HZ-1245 | ≤1 ng/mL EC50 | >95% |
| IL-29 | HZ-1156 | ≤5 ng/mL EC50 | >95% |
| Lefty-1 | HZ-1109 | ≤40 ng/mL EC50 | >95% |
| LIF | HZ-1292 | ≤0.2 ng/mL EC50 | >95% |
| M-CSF | HZ-1192 | ≤4 ng/mL EC50 | >95% |
| Noggin | HZ-1118 | ≤15 ng/mL EC50 | >95% |
| Oncostatin M | HZ-1030 | ≤1 ng/mL EC50 | >95% |
| PDGF-aa | HZ-1215 | ≤10 ng/mL EC50 | >95% |
| PDGFbb | HZ-1308 | ≤3 ng/mL EC50 | >95% |
| Pleiotrophin-PTN | HZ-1278 | N/A | >95% |
| pro-IGF-II | HZ-1161 | ≤50 ng/mL EC50 | >95% |
| SCF | HZ-1024 | ≤25 ng/mL EC50 | >95% |
| Sonic Hedgehog (SHH) | HZ-1306 | ≤350 ng/mL EC50 | >90% |
| TGF beta 1 | HZ-1011 | ≤0.5 ng/mL EC50 | >95% |
| TGF beta 2 | HZ-1092 | ≤0.5 ng/mL EC50 | >95% |
| TGF beta 3 | HZ-1090 | ≤0.5 ng/mL EC50 | >95% |
| Thrombin | HZ-3010 | N/A | >95% |
| TNF alpha | HZ-1014 | ≤0.5 ng/mL EC50 | >95% |
| TPO | HZ-1248 | ≤5 ng/mL EC50 | >95% |
| VEGF 121 | HZ-1204 | ≤15 ng/mL EC50 | >95% |
| VEGF 165 | HZ-1038 | ≤5 ng/mL EC50 | >95% |
| Wnt3A | HZ-1296 | ≤20 ng/mL EC50 | >90% |
* 주문, 견적 문의는 지사/대리점으로 연락 주세요.
- 지사/대리점 안내Opens in a new tab

